To find the molecular mechanisms of phagocytic cells, macrophages and neutrophils as key players in the immune system
What are phagocytic cells?
Phagocytes belong to white blood cells, which assume the critical role of host defense. Especially, neutrophils and macrophages have high motility: they migrate to the infection focus, engulf microorganisms and kill them using bacteriocidal enzymes or ROS production as if a single-cell organism.
Goal of our research
Phagocytes defend our body against foreign invasion, but hyperfunction or excess destruction of the cells/tissues sometimes induces severe diseases. We are aiming at the research leading to solution of the hematological/immunological disorders such as infection-related diseases and autoimmune diseases, and contribution to the development of the novel therapeutics by taking advantage of cell and gene technology.
Research projects
(1) Molecular mechanism of complement-mediated phagocytosis
Phagocytosis is a central event in the innate immunity and is triggered by the association between the parts of pathogens as ligands and receptors on the membrane of phagocytes. Particularly, the complement-mediated phagocytosis is accomplished by recognizing complement components bound to the pathogens by the complement receptors. We have studied this system and found that (1) a tyrosine kinase Syk is a key molecule of phagosome formation (Blood. 2006;107(11):4554-62. ) , and (2) Rab27a, a small GTPase of the Rab family, acts as a regulator of the complement-mediated phagocytosis by controling both actin dynamics and pathogen engulfment (J Biol Chem. 2011;286(7):5375-82 .).
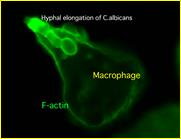 Furthermore, we are interested in searching for the molecular mechanisms by which macrophages inhibit the escape of microorganisms from phagosomes using Candida albicans.
Furthermore, we are interested in searching for the molecular mechanisms by which macrophages inhibit the escape of microorganisms from phagosomes using Candida albicans.
(2) Elucidation of the mechanism of bone resorption by osteoclasts
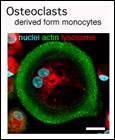 Bone is continuously degraded and reconstituted. Osteoclasts, specialized cells in the bone marrow, degrade the bone matrix but the precise mechanism of osteolysis has not been fully understood. To find a molecular-target for osteolytic disorders such as osteoporosis and rheumatoid arthritis, we performed in vitro osteolysis assay using monocyte-derived osteoclasts and found that an energy molecule ATP induces osteolysis via P2X7 receptor-mediated signaling and that deacetylation of alpha-tubulin is essential for the whole process of osteolysis under the control of a tyrosine kinase Syk (Genes Cells. 2009; 14: 871-84. ) .
Bone is continuously degraded and reconstituted. Osteoclasts, specialized cells in the bone marrow, degrade the bone matrix but the precise mechanism of osteolysis has not been fully understood. To find a molecular-target for osteolytic disorders such as osteoporosis and rheumatoid arthritis, we performed in vitro osteolysis assay using monocyte-derived osteoclasts and found that an energy molecule ATP induces osteolysis via P2X7 receptor-mediated signaling and that deacetylation of alpha-tubulin is essential for the whole process of osteolysis under the control of a tyrosine kinase Syk (Genes Cells. 2009; 14: 871-84. ) .
(3) Elucidation of the mechanism of NET formation
Neutrophils play a crucial role in host defence. In response to a variety of inflammatory stimulation, they form neutrophil extracellular traps (NETs) . NETs are extracellular 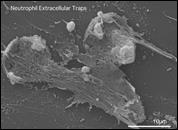 structures composed of chromatin fibers decorated with antimicrobial proteins, and developing studies indicate that NETs contribute to extracellular microbial killing. While the intracellular signaling pathways that regulate NET formation remain largely unknown, there is growing evidence that generation of reactive oxygen species (ROS) is a key event for NET formation. To investigate the role of Rab27a in neutrophil function in detail, both primary human neutrophils and neutrophil-like differentiated HL60 cells were treated with PMA, and NET formation process was assessed by measurement of release of histone H3 into the medium, citrullination of the arginine in position 3 of histone H4 and chase of the nuclear change of the living cells in the co-existence of both cell-permeable and -impermeable nuclear indicators. PMA-induced NET formation occurred sequentially in both neutrophil-like differentiated HL60 cells and primary neutrophils, and Rab27a-
structures composed of chromatin fibers decorated with antimicrobial proteins, and developing studies indicate that NETs contribute to extracellular microbial killing. While the intracellular signaling pathways that regulate NET formation remain largely unknown, there is growing evidence that generation of reactive oxygen species (ROS) is a key event for NET formation. To investigate the role of Rab27a in neutrophil function in detail, both primary human neutrophils and neutrophil-like differentiated HL60 cells were treated with PMA, and NET formation process was assessed by measurement of release of histone H3 into the medium, citrullination of the arginine in position 3 of histone H4 and chase of the nuclear change of the living cells in the co-existence of both cell-permeable and -impermeable nuclear indicators. PMA-induced NET formation occurred sequentially in both neutrophil-like differentiated HL60 cells and primary neutrophils, and Rab27a-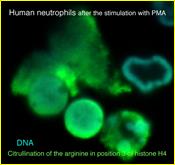 knockdown clearly inhibited NET formation in association with reduced ROS production. We also found that serum-treated Candida albicans triggers NET formation in a ROS-dependent manner, and that Rab27a-knockdown inhibits this process as well.
knockdown clearly inhibited NET formation in association with reduced ROS production. We also found that serum-treated Candida albicans triggers NET formation in a ROS-dependent manner, and that Rab27a-knockdown inhibits this process as well.
Our findings demonstrate that Rab27a plays an important role in NET formation induced by both Candida albicans infection and PMA treatment by regulating ROS production. (PLoS One. 2014 9(1):e84704. )

 Top Page
Top Page